Insight into How Cells Get Signals from Physical Senses Could Lead to New Disease Treatments
UMD-led study opens door to understanding cell’s sense of touch
The body’s cells are constantly receiving and reacting to signals from their environment. A lot is known about how a cell senses and responds to chemical signals, or biomolecules, such as COVID-19. But little is known about how signals from the physical environment, like touch, temperature or light, direct a cell’s activity. Understanding that process could lead to new ways of treating cancer and other disease.
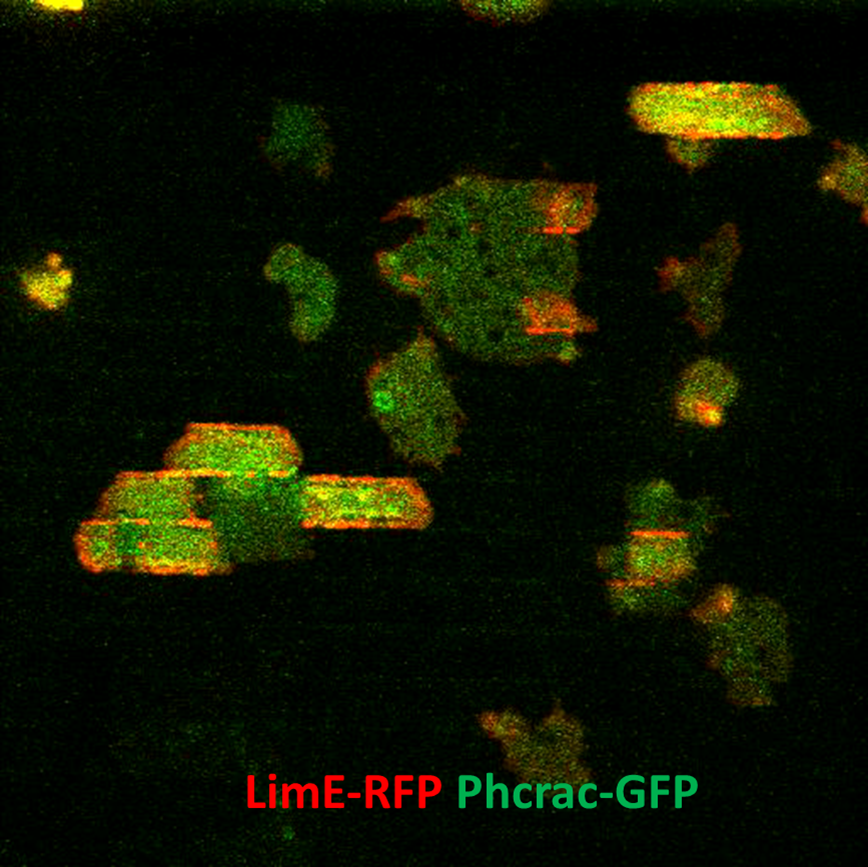
A new study published May 1, 2023 in the Proceedings of the National Academy of Sciences by a University of Maryland-led Multidisciplinary University Research Initiative (MURI) funded by the Air Force Office of Scientific Research has opened the door to seeing how cells react to physical signals.
“We elucidated a cell's sense of touch,” said Wolfgang Losert, a professor of physics at UMD and a team leader of the study. “We think how cells sense the physical environment may be quite distinct from how they sense the chemical environment. This may help us develop new treatment options for conditions that involve altered physical cellular environments, such as tumors, immune disease and wound healing.”
A major difference between chemical and physical signals is size. Chemical signals are 100,000 times smaller than the width of a human hair. Physical cues are the heavyweights in the ring.
“We looked at how cells sense crucial physical cues from their environment that are on the order of 100 times larger than chemical signaling molecules,” said Losert, who also has a joint appointment in UMD’s Institute for Physical Science and Technology (IPST).
“We’re really answering a kind of long-standing mystery of how cells react to cues in their environment that are on a physical rather than chemical-size scale,” said paper co-author and MURI team member John T. Fourkas, a professor in UMD’s Department of Chemistry and Biochemistry with a joint appointment in IPST.
The MURI team studied the major players in a cell’s interaction with its physical environment: the cytoskeleton, a network of proteins that surround a cell and acts as a direct sensor of the physical environment; actin, the protein that keeps cells connected; and the cell’s signaling pathways.
Qixin Yang (Ph.D. ’22, physics), who led the experiments and analysis for her Ph.D. research at UMD, said, “I think our work related to the cytoskeleton shows that it plays an important role in sensing physical cues, like pain.”
The MURI team found that the networks that guide cell migration are upstream for chemical sensing and downstream for physical, topographic sensing; and that actin is the direct sensor for both types of signals.
“I think this is the first real crucial confirmation that actin itself is the sensor and that the waves are really where they are in the sensing pathway, not way downstream, but up front and center,” Fourkas said.
“Our findings reveal that, in much the same way that patterns of waves in the ocean allow an expert surfer to understand the undersea topography, the so-called ‘mechano-chemical’ waves in cells are key in sensing signals from their physical environment that are much larger than single proteins,” Losert said. “That has implications for how you might design physical interventions to change the behavior of cells.”
For instance, previous research by a co-author of this study, Peter Devreotes of Johns Hopkins University, found that actin dynamics were different for cancer cells considered most invasive.
“Understanding how drugs impact waves is an important additional piece of information that may be used in making decisions on treatment options,” Losert said. “I see our study also providing pointers on how you can improve the ability of immune cells to be guided to their target.”
The MURI team is made up of researchers in physics, chemistry, biology, bioengineering and dermatology from the University of Maryland and several other institutions.
###
In addition to Losert, Fourkas and Yang, UMD chemistry graduate student Matt Hourwitz was a co-author of the paper.
The paper, “Nanotopography modulates intracellular excitable systems through cytoskeleton actuation," was published in PNAS on May 1, 2023.
This research was supported by the Air Force Office of Scientific Research (Award No. FA9550-16-1-0052). This story does not necessarily reflect the views of this organization.
Written by Ellen Ternes